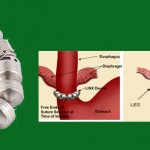
This page is being provided to help those who are looking for documentation to support insurance coverage for the LINX magnetic augmentation device. If you are pursuing an appeal of a denial of coverage because your insurance considers it experimental or investigational, these resources may be of assistance. We assume you know about Torax’s site linxforlife.com
If you have had success with appeals or just approval and would like to provide documentation for others, we welcome this.
Successful approvals or appeals should include the company, dates, and plan information (or a link to it) so that others may match to their situation. However, any information that is provided will be redacted to anonymize any personal information. Contact linx@chena.org if you would like to contribute to this community or if you have questions.
We offer no medical advice or any guarantees, just a chance for patients to take control of and advocate for their own health needs.
As of 2018, there has been continued success in getting insurance approvals though it often takes an external appeal. It seems like it will get harder for health plans to refuse this, partly because of the confirming efficacy and safety studies now completed and the continuing demonstrated impacts of the proton pump inhibitor drugs, from bone fractures, kidney damage, dementia and more.
Federal Drug Administration
- Jan-Mar 2012 meeting Executive Summary
- FDA Approval March 2012
- Press Release
- Approval Order
- Summary and Labeling
- Transcript of panel reviewing device Jan. 11, 2012.
This transcript is a long pdf file, but a most interesting read. See particularly Federico Soldani’s testimony beginning at p. 108 in which he indicates that further study is only to demonstrate ‘continued safety and effectiveness’, saying essentially, that, if the FDA gave pre-marketing approval (which they did), that safety and effectiveness WERE in fact demonstrated. Regarding the lack of controlled trials that the insurance companies have been touting in their denials, see p. 125 as to why it would be inappropriate. - Here’s a pdf of the slides referenced in the transcript.
- If you want the multimedia version, here it is, but it’s 66 mb.
Presentations
Here’s a concise presentation by renowned LINX expert Dr. John Lipham, of USC Keck Surgical Center.
Studies
You can find most of the studies on line, or at least the abstracts. I’ve noted the recent studies.
- Long-term Outcomes of Patients Receiving a Magnetic Sphincter Augmentation Device for Gastroesophageal Reflux Clinical Gastroenterology and Hepatology, Ganz et.al., 2016 – this is the 5 year required FDA study, confirming safety and efficacy. If this doesn’t do it for getting approvals, I don’t know what will.
- LINX-Nissen comparison, Annals of Thoracic Surgery, Louie, et.al., 2014
- 1000 Patients Safety, Int’l Society for Diseases of the Esophagus, Lipham, et.al., 2014
- Multicenter observational study 249 patients, Surgical Endoscopy, Riegler, et.al., 2014
- Six year 100 patient study, Journal of the American College of Surgeons, Bonavina, et.al., 2013
- 100 Patients Evaluation before and after, 3 year of 5 year pivotal study, New England Journal of Medicine, Ganz, et.al., 2013
- LINX safety and efficacy at 4 years, Surgical Endoscopy, Lipham, et.al., 2012
- Pivotal 5 Year Ganz Study – Long-Term Outcomes of Patients Receiving a Magnetic Sphincter Augmentation Device for Gastroesophageal Reflux, Clinical Gastroenterology and Hepatology, Ganz, et.al. 2015
- Pilot 5 Year Saino study – Magnetic Sphincter Augmentation
for Gastroesophageal Reflux at 5 Years: Final Results of a Pilot Study Show Long-Term Acid Reduction and Symptom Improvement, Journal of Laparoendoscopic & Advanced Surgical Techniques, Saito, et.al. 2018 - Worldwide Experience with Erosion of the Magnetic Sphincter Augmentation Device – Journal of Gastrointestinal Surgery – Alicuben, Bell, Jobe, Buckley III, Smith, Graybeal, Lipham, 2018
- Guidelines for GERD-Katz.et.al-Am.Journal.Gastroenterolog 02-13y – aspects of their collected recommendations don’t make sense to me, for example saying that osteoporosis isn’t a reason to stop PPI’s or that failure to respond to PPI’s doesn’t suggest surgical intervention. Maybe someone else can figure out these stances.
- Nonmedical Therapeutic Strategies for Non-Erosive Reflux-Maradey-Romero-J Clinical Gastro 08-2014 – while this doesn’t address erosive esophagitis treatment, they do briefly discuss the LINX>
- Australian Health Policy Presentation-Aug. 2013 – the publisher caveats they don’t endorse this, but it might be helpful to see what the prevailing Australian view was in mid 2013.
- Lower Esophageal Sphincter Augmentation for Gastroesophageal Reflux Disease:
The Safety of a Modern Implant, Journal of Laparoendoscopic & Advanced Surgical Techniques, Smith, Ganz, Lipham, Bell, Rattner. 2017
.
Approvals
Because the FDA approved the LINX as being safe and efficacious, federal health care programs such as Medicare and Medicaid accept this body’s ruling. Medicare-Medicaid-LINX Coding and Billing Guide 2014 implemented Jan. 1, 2014.
The State of Michigan has a number of public appeals on record. As of Feb. 2014, they all seem to be successful in overturning the insurance companies’ denials. In general, there are inconsistent results with external reviewers, a real crap shoot and that’s not right. One industry insider told me he thought it was criminal.
- Blue Cross Blue Shield Feb. 10, 2014
- Priority Health April 8, 2014
- Blue Cross Blue Shield May 20, 2014
- Blue Cross Blue Shield May 21, 2014
- Here’s another granting an appeal by the employer over Aetna, their 3rd party health care administrator. The employer is a law firm. You think they know something about liability maybe?
One LINX recipient told me about an informal appeal that was granted. The story goes is that the insurance representative called the patient a couple days after the patient had put in for pre-authorization. He told her it was denied because it was experimental-investigational. The patient told he was an idiot, indicating it had been approved by the FDA and referred him to toraxmedical.com. A few days later, he called back and it was approved. Not the normal bureaucratic extended process.
I do have a handful of pre-authorization approvals that didn’t have to go through the appeals process. Let us know if you would like copies for your own case.
More info as I get it.